Inside an Individual’s Physiological Signature
Originally published in Medical Device & Technology
6 min read
![]() physIQ
:
August 14, 2020
physIQ
:
August 14, 2020

Originally published in MATTER
Throughout the COVID-19 pandemic, businesses across healthcare have faced the challenge of retooling and pivoting their solutions to focus on the fight against COVID-19. But for MATTER startup physIQ, no pivoting, revising or overhauling was necessary: The company’s predictive analytics technologies were already uniquely suited to meet several of the needs and use cases of COVID-19 — and ready to deploy.
Founded in 2013 and based in Chicago, physIQ improves health outcomes by applying artificial intelligence to real-time physiological data from wearable sensors. This model allows physIQ to detect subtle, often pre-symptomatic changes to someone’s physiology — something that is especially important to healthcare providers as they work to understand how COVID-19 acts across different individuals.
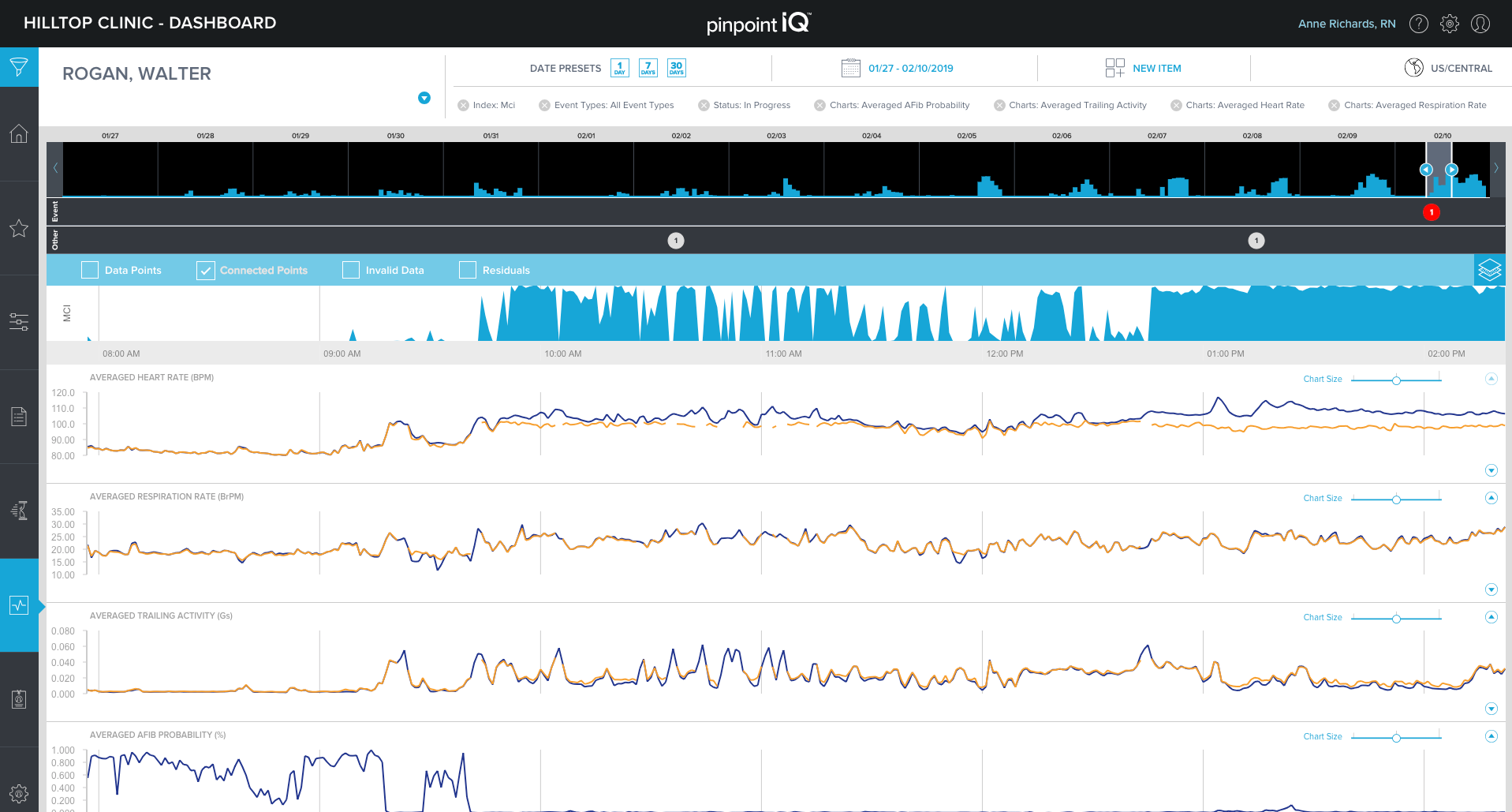 A look into physIQ’s pinpointIQ portal.
A look into physIQ’s pinpointIQ portal.
2020 is not the first time that physIQ has used their technology to respond to a pandemic. In 2015, the company worked with the Centers for Disease Control, the United States Agency for International Development and Scripps Health to provide high-acuity continuous monitoring for hospitals fighting the Ebola outbreak in Sierra Leone. As a result of that project, physIQ collected more than one million hours of annotated physiological data and published a study validating that their solution could be used to streamline care in virtually any healthcare setting, anywhere in the world.
It was those successes five years ago that paved the way for physIQ to take on two major COVID-19 projects: In May, the company teamed up with the Department of Defense (DoD) and the Henry Jackson Foundation on a clinical study to collect and analyze wearable sensor data from military service members with the goal of better understanding how COVID-19 progresses. In June, they announced a partnership with Chicago Medical Society and University of Illinois Health (UIH), a Chicago-based health system, to help protect frontline COVID-19 healthcare workers and high-risk patients.
We connected with three people closest to these projects, Gary Conkright, founder and CEO, Karen Larimer, director of clinical development (UIH project) and Stephan Wegerich, chief science officer (DoD project), to learn more about each project, what they learned and what their advice is for other startups looking to collaborate with large organizations.
Responses have been slightly edited for length and clarity.
Karen: “The way everyone in healthcare delivery is looking at COVID-19 is ‘all hands on deck’. They want to understand what they can do to attenuate the number of people getting sick and the number of people dying. One of the opportunities we’re exploring at UIH is helping them identify how they might ensure that their own healthcare workers don’t get as sick once they are exposed — whether that’s in the workplace setting or at home. Once they are sick, they want to be able to monitor them in a way that helps them avoid any adverse health outcomes.”
Gary: “This work is important because at UIH, their staff demographics are representative of the community they serve. The healthcare workers and staff members of UIH that are being monitored with our pinpointIQ product today will demonstrate the product’s benefit in addressing the health disparities that are once again being highlighted by COVID.“
Stephan: “The DoD project is interesting because it’s a hybrid of observational and interventional — we’re monitoring people, but at the same time we’re tasked with collecting data for future research. The idea is to gather as much wearable sensor data from service members who have already positively tested for COVID-19, as well as from people at risk for contracting the virus. Along with the wearable sensors that we provide, blood samples and other information is being collected to ultimately help us understand COVID-19 better and uncover why it is that certain people seem to handle the disease better than others. We started off with two sites, but ultimately we’re looking to expand the project to monitor thousands of service members.”
Karen: “I would say that from the first email regarding the project to the first patient enrolled, it was a span of only 50 days. If you have experience with standing up platforms, enrolling people and reconfiguring workflows, that’s really fast. We joke about it now, but we had this ‘Open 24 hours a day, seven days a week’ kind of philosophy. Our collaborators were used to that being ER workers, and we all just wanted to get it going. Eye on the prize. And with that kind of attitude, it’s amazing how much you can get done.”
Stephan: “I had a very similar experience. By comparison, for the 2015 Ebola project, from the time we submitted an IRB to the time we started was probably around nine months — and even after that, we still had contracting work to figure out. In contrast, from the time of the protocol to the start of enrollment was on the order of weeks. With this project, we realized we needed to get going right away. Everyone involved agreed on what we’re going to do and what the contract would cover, and so the study began even before all of the components contracting were complete.”
Karen: “I was very tuned in and sensitive to the healthcare professionals, specifically the advanced practice nurses (APN), who would be using the product. We never want it to feel like too much additional work — and Stephan and I are also always worried about false positives and false negatives. But our analytics were just stellar and the APNs didn’t really have any complaints about the workflow.
“As for the patients, we had many saying that they just loved, loved, loved using the product. Instead of complaining about being hassled by wearing a patch — which is what we always worry about — they didn’t want to let go of it. One patient who was admitted to the ER actually refused to take her patch off because she wanted to continue to monitor herself through our platform for the entire time that she was in the hospital. She was just recently discharged and she continues to monitor at home.”
Stephan: “I’m always looking at the data, and in that particular case at UIH, it’s almost textbook for us — if you had to put something in a textbook about our personalized analytics, that would be it.”
“For the DoD project, we don’t have the outcome information yet, but I monitor the data all the time. What’s really interesting about the subjects in this project so far is that they’re all pretty healthy people, and typically asymptomatic. When you look at their data, you can clearly see when they’re doing interval training, for example. They’re COVID-19 positive and they’re still managing to do interval training, but then I’ll notice that on certain days their recovery times appear to be much longer. I can’t wait to get the other information to see if there are correlations with outcomes — the subjects might not even notice the additional fatigue caused by the virus, but it’s right there in the cardiopulmonary signature.”
Gary: “Both of these projects have been great opportunities for us to showcase what AI, when applied to physiological data from wearables, can do. And I think these results will basically accelerate our trajectory, because they’re both a vivid demonstration of how technology can be deployed — and should be deployed — to allow both clinical studies and healthcare delivery leverage the power of technology.”
Gary: “We will get through this. And when we look back at this point in time, I believe we’re going to be able to say that COVID-19 actually accelerated the whole digital health ecosystem. I think we’re going to be where we anticipated being in five years later this year — because you can’t put Pandora back in the box. Everyone has now realized that you can monitor patients at home with very high fidelity data and very high fidelity analytics that enable care delivery professionals, nurses and doctors to take better care of those patients.
“The same is true for clinical studies. The virtual clinical trial is upon us. Clinical study sponsors are going to be able to get 24/7 data — real physiological data and real-time patient-reported outcomes so that they can do a lot more than they’ve ever done before and a lot faster than they’ve done it before.”
Karen: “We’ve had great success combatting this surprise use case that just popped almost out of nowhere. I’d like to see what happens if we use what we’ve learned to tackle other problems — chronic issues with frequent readmissions like heart failure, COPD or pneumonia — with the same immediacy and attitude.”
Stephan: “Ironically, our system has always been capable of responding to a situation like this. It’s our understanding of what that response would look like that has been the struggle, and now we know. It’s fascinating.”
Karen: “What made the UIH project possible was the buy-in across both organizations and at all levels of leadership. physIQ was all in and so was UIH. What helped the most was not only that there were people at the highest levels of leadership that were champions of the project, but that there was also leadership buy in at the mid-level and with the folks actually executing on the platform. They were involved in decision making and were available to troubleshoot and communicate easily.”
Stephan: “You want to connect with people that are very driven and have the same goals as you. In both of these projects, there are multiple champions on the other side of the partnership that really buy into this. They’re not being mandated to do this work — they’re doing it because they really believe in it.”
Gary: “Chicago has some great clinical partners and organizations — MATTER being one of them — that can connect the dots for a startup. What we all seek as startup CEOs is acceleration and getting to the right individual within the right organization to make the pitch so we can explain what we’re capable of doing. These warm introductions are worth their weight in gold.”

Originally published in Medical Device & Technology

Originally published in Pixel Scientia Labs

Originally published in Crain's Chicago